The charge of a proton and an electron are equal in magnitude yet opposite in sign. Both protons and neutrons have a mass of 1 while electrons have almost no mass.

How To Calculate Atomic Mass Teaching Chemistry Chemistry Relative Atomic Mass
- Each proton has a positive electrical charge.
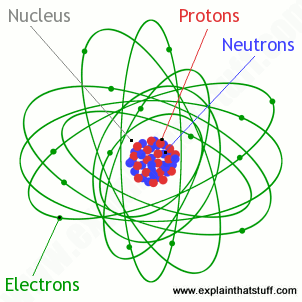
. The number of electrons in each elements electron shells particularly the outermost valence shell is the primary. Number of neutrons mass number - atomic number 23 - 11 12. The mass of a proton is 167262158 10.
Which one of the following statements is true 19. Which one of the following does not contribute to the size radius of an atom 17. List the properties of protons neutrons and electrons with respect to mass and electrical charge.
Start studying Properties of Protons Electrons and Neutrons. Neutron is an uncharged particle of the nucleus of all atoms EXCEPT hydrogen. Neutron does not effect the chemical properties of an element.
Therefore they have a no net charge. The chemical properties of the atom are determined by the number of protons in fact by number and arrangement of electrons. If the charge is positive there are more protons than.
The negative charge of one electron balances the positive charge of one proton. Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. The charge on proton is positive 160217646 10 -19 coulombs.
112 rows Sodium has 11 protons 12 neutrons and 11 electrons. Number of protons 11. The number of neutrons can be obtained by the difference between mass number and atomic number.
3 rows Protons and neutrons have approximately the same mass but they are both much more massive than. Atoms Protons And Electrons images similar and related articles aggregated throughout the Internet. The proton has a mean square radius of about 087 1015 m or 087 fm and it is a spin ½ fermion.
Atoms are made of protons neutrons and electrons. Ion is an atom that has gained or lost one or more electrons thus becoming positively or negatively charged. - Each electron has a negative electrical charge.
An atom with three protons is a lithium atom an atom with five protons is a boron atom an atom with six protons is a carbon atom. The mass of a neutron is 16749 x 10 -27 kg. Properties of Neutrons Neutrons together with protons are the constituents of atomic nuclei.
A neutron is only stable when bou. The neutron is slightly heavier than a proton and can therefore decay into the lighter particles a proton an electron and an anti-electron-neutrino. Protons neutrons and electronsAs summarized in Table 21 protons are positively charged neutrons are uncharged and electrons are negatively charged.
So here number of protons and electrons are equal to 19. 3 protons 3 electrons and 4 neutrons. Which of the following determines the chemical properties of an atom 18.
It has a positive electric charge 1e and a rest mass equal to 167262 1027 kg 938272 MeVc2 marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. Number of electrons 11. The list goes on.
Over 5 electrons so its negatively charged cscscsafewqfdewqdrwe. On the same logic we can get the number of protons neutrons and electrons in each pair of isotopes For option B. As summarized in Table 2.
Which of the following statements is true a. If scientists count four protons in an atom they know its a beryllium atom. Answer 1 of 2.
Thus number of neutron is eqrm 40 - 19. Electrons can be shared or transferred among atoms. Proton is a positive particle.
Name Helium Protons 2 Neutrons 2 Electrons 2 Atomic Number Z 2. Protons and neutrons 16. Protons are posotively charged and electrons are negatively charged the neutrons are not on rubber there are less protons.
Learn vocabulary terms and more with flashcards games and other study tools. An ion has an unequal number of protons and electrons. The configuration of these electrons follows from the principles of quantum mechanics.
21 Electrons Protons Neutrons and Atoms All matter including mineral crystals is made up of atoms and all atoms are made up of three main particles. Every solid liquid gas and plasma is composed of neutral or ionized atoms. A neutral atom has the same number of protons and electrons charges cancel each other out.
Protons and neutrons are present in the nucleus of the atom. 1 protons are positively charge neutrons are uncharged and electrons are negatively charge. The atomic mass is 7 and the atomic number is 3 which means the number of protons is 3 and the number of neutrons is 4 and as it has no charge then the number of electrons is equal to the number of protons that is 3.
5 rows They are present in the outer shells within an atom and orbit the positively charged nucleus in. Atoms have an equal number of protons and electrons. Electrons and protons d.
Popular Posts
Search Here
Arsip
Categories
- 1500
- 1959
- 1968
- 1984
- 1985
- 1989
- 1992
- 2002
- 2007
- 2008
- 2013
- 2014
- 2015
- 2020
- 2021
- 2042
- 2122
- 21st
- 3
- 40mg
- 5
- 500
- a4
- about
- accept
- achievement
- adams
- adoption
- adversity
- advertising
- advisors
- affirmation
- Affleck
- African
- after
- air
- airport
- ajax
- Aktenzeichen
- aladdin
- alain
- alfa
- algerie
- all
- allen
- alone
- Alparslan
- american
- americas
- and
- Andrew
- Angela
- angeles
- anime
- Annalena
- anni
- annie
- anniversary
- announcement
- another
- Anthony
- antigen
- Antwerp
- appointment
- appreciation
- april
- arabic
- Arfa
- argument
- arouca
- Arsenal
- artvin
- aston
- atletico
- Atlético
- audi
- australian
- author
- auto
- awake
- baby
- baccarat
- back
- Baerbock
- bahasa
- bang
- baptism
- Barcelona
- baskerville
- Battlefield
- bayern
- bc
- beach
- beatles
- beau
- beautiful
- beauty
- belmondo
- belt
- benfica
- Benjamin
- benz
- best
- BETTINO
- biafra
- bible
- birthday
- blessings
- blues
- bmw
- Boca
- bodybuilder
- bohemian
- Bohusläningen
- Borussia
- boseman
- boulevard
- bowls
- boyfriend
- bracelet
- brandon
- braut
- braves
- brazil
- Brendan
- Brentford
- brewers
- Brian
- Brighton
- britney
- buat
- bugatti
- bugs
- Buienradar
- building
- bulls
- bullying
- bunny
- Büyük
- c50
- cabrio
- Cadet
- cadillac
- cafe
- caicos
- caida
- caída
- calculator
- calendar
- Camping
- Campionato
- Canadiens
- canal
- cant
- captions
- car
- care
- carolina
- Carolyn
- cars
- Carter
- cast
- cause
- cayenne
- Celebrity
- Central
- century
- chadwick
- chain
- championnat
- Champions
- changed
- changes
- charles
- Charlie
- charlotte
- cheapest
- chelsea
- cherokee
- chevrolet
- chevy
- chiwetalu
- chocolate
- chr34
- christian
- christmas
- Christopher
- chrysler
- cincinnati
- cite
- City
- Claire
- Classement
- classroom
- clues
- Cluj
- college
- colo
- colonel
- commitment
- community
- companies
- conditioner
- conference
- convert
- convertible
- cooke
- cool
- coping
- coskuner
- costco
- country
- couple
- cover
- covid
- Craig
- Craiova
- CRAXI
- crazy
- cresco
- crossword
- crown
- crv
- Crystal
- Cuidar
- current
- currie
- customers
- cute
- cyclisme
- dacia
- daddy
- daily
- dairyland
- Daniel
- dark
- darling
- daughter
- days
- dead
- dealers
- deals
- death
- debat
- deceit
- december
- decorations
- decreasing
- deep
- delon
- denzel
- depression
- dept
- Deputy
- detect
- deville
- diary
- diego
- direct
- dirt
- distance
- diwali
- djibouti
- djokovic
- do
- documentario
- dodge
- donde
- dont
- Dortmund
- down
- download
- drawing
- dream
- duster
- duty
- dyer
- easter
- editable
- edo
- edward
- egypt
- ehsaan
- einstein
- Elden
- electrons
- elon
- emile
- Emmanuel
- Emmys
- emoji
- encouragement
- ene
- enemy
- england
- english
- enjoying
- Enstroga
- episode
- éric
- erling
- Espanyol
- essence
- eventually
- everdeen
- Evergrande
- everyone
- ex
- example
- eyebrow
- Fabre
- fake
- familj
- family
- famous
- farm
- father
- faye
- FC
- felix
- ferdinand
- festival
- fiat
- fifa
- film
- financial
- fine
- first
- flag
- flexible
- Florence
- florida
- flowers
- font
- fool
- for
- forgot_about_dre
- formel
- foto
- Foy
- francais
- france
- Francis
- freddie
- free
- freight
- french
- friend
- friends
- friendship
- from
- Fugueuse
- funny
- fury
- gabby
- Gabriela
- gaddafi
- Gakara
- Galaxy
- game
- games
- gaming
- garage
- garing
- Garson
- Gary
- gatsby
- general
- george
- Gerda
- Gergely
- german
- Giants
- gibran
- girlfriend
- girls
- give
- giving
- gli
- goals
- goethe
- golf
- good
- goodbye
- Górnik
- graduates
- grand
- grandma
- grandson
- granite
- grateful
- gratitude
- great
- greek
- Green
- grief
- grimes
- group
- gta
- guerra
- guilty
- Gulyás
- hair
- håland
- happiness
- happy
- hard
- hardship
- Hatem
- having
- hd
- head
- heart
- height
- hemingway
- herodotus
- highlight
- highlights
- hindi
- histoyr
- hjelmer
- holden
- holiday
- home
- homestead
- honda
- honesty
- honor
- horse
- hounds
- how
- humor
- hurricane
- hurts
- husband
- hyundai
- iconic
- idade
- IGPR
- images
- imdb
- importance
- index
- india
- infiniti
- infinity
- inggris
- inmates
- insignia
- inspirational
- insurance
- Inter
- international
- iphone
- ireland
- Irene
- is
- isko
- islamic
- italicized
- iverson
- izle
- jack
- jacket
- jagger
- jaguar
- jaguars
- James
- janoski
- Jasper
- Javier
- jealousy
- Jean
- jeep
- jeeps
- jennifer
- jesus
- jewish
- joao
- Jobs
- jodoh
- jogger
- Johannes
- john
- joker
- Joshua
- jp
- Juniors
- just
- Juventus
- kata
- katniss
- kaufen
- kemalpaşa
- Kena
- khalil
- kickboxing
- kids
- king
- kipling
- kiss
- klink
- korean
- Kyle
- la
- Laatste
- labs
- lamborghini
- land
- landscape
- language
- lanus
- last
- lawn
- lawrence
- Le
- League
- leah
- learning
- leave
- Lech
- Łęczna
- leeds
- legg
- leibovitz
- Leicester
- lennon
- lessons
- lexus
- liberal
- life
- lifes
- lifestyle
- limitations
- lincoln
- literature
- little
- live
- Liverpool
- llorando
- locust
- london
- long
- looking
- Lopez
- lord
- los
- lost
- love
- lovers
- loyal
- luther
- Lyon
- lyrics
- macey
- Macron
- madrid
- magic_quotes_gpc
- makeup
- manchester
- manning
- manu
- marathi
- Maria
- mark
- Market
- marley
- maroney
- married
- marti
- martin
- maserati
- Massimo
- MasterChef
- Matrix
- matthew
- mayer
- mazda
- McDowell
- me
- meaning
- medvedev
- meet
- melenchon
- Memo
- mercury
- Merkel
- merry
- message
- messages
- messed
- messer
- Meta
- mick
- midlothian
- midsummer
- midtown
- Milan
- mindler
- mini
- Mino
- mockingbird
- modern
- monde
- monterey
- moreno
- morgan
- morning
- mortgage
- mother
- motivate
- motivational
- motorcycle
- mourning
- movie
- movies
- Movistar
- mpg
- multiair
- Munich
- murals
- musk
- nacio
- Nations
- native
- nature
- near
- need
- neil
- never
- newest
- newlyweds
- news
- nieuws
- night
- Nightingale
- nj
- noah
- not
- nothing
- Nouvelles
- nyaman
- obama
- ocean
- of
- oficial
- oleksandr
- olympia
- on
- once
- opel
- open
- osho
- outback
- outline
- pacar
- Packers
- page
- pageant
- paint
- Palace
- palin
- pandemic
- pants
- paper
- parents
- park
- partido
- parts
- passing
- past
- patience
- paul
- pennington
- people
- peppermill
- person
- personality
- peru
- Peter
- petersburg
- peterson
- petito
- peugeot
- philadelphia
- philosophical
- pickup
- picture
- pierre
- più
- player
- plymouth
- pontiac
- porsche
- positive
- positivity
- post
- Poste
- praise
- predictions
- President
- pret
- prettier
- price
- prince
- printable
- problems
- prom
- properties
- protest
- proton
- protons
- pursuit
- pyramids
- pzev
- q50
- quad
- quale
- questions
- quote
- quoted
- quotes
- r56
- Raiola
- range
- rapid
- ratings
- reading
- Real
- receh
- Recess
- record
- Reeve
- reference
- registration
- relationship
- relationships
- release
- Remigi
- Rennes
- repair
- Republic
- Reserva
- resorts
- resultado
- resurrection
- Resurrections
- Reum
- review
- rhapsody
- Ring
- Rittenhouse
- River
- rmx250
- Rodgers
- Rogers
- rolls
- romantis
- romeo
- root
- rosa
- rose
- rover
- rowe
- rupi
- rússia
- Ruto
- rv
- s22
- Sabatini
- sales
- saliva
- salons
- sample
- samsung
- santa
- Santiago
- sarasota
- sayings
- says
- Scharten
- Schouten
- season
- Selçuklu
- self
- sense
- september
- Serie
- series
- service
- Sevilla
- sezon
- Shailene
- shakespeare
- Shandro
- Sheriff
- shes
- shiffrin
- shipping
- shoes
- short
- should
- show
- side
- sign
- simple
- sister
- sisters
- site
- SK
- sleep
- smith
- society
- solo
- someone
- Sommerhaus
- songs
- sorry
- soulmate
- soup
- south
- Southampton
- sp500
- Space
- spain
- spanish
- spears
- special
- speed
- Spezia
- spider
- Spirits
- sporting
- spot
- spray
- staffing
- stanley
- Stars
- status
- Steve
- stock
- strength
- Strolz
- students
- Stuttgart
- Stuyven
- subaru
- subordinate
- success
- suddenly
- summary
- summer
- sunset
- sunshine
- superbirds
- superior
- support
- Suriname
- Sutcliffe
- suv
- suzuki
- sydney
- symbol
- syriac
- tagalog
- tahoe
- tahun
- tallahassee
- tamil
- taplin
- tarquin
- tax
- tb
- teachers
- tell
- teotihuacan
- term
- test
- texas
- text
- texture
- than
- thank
- thanksgiving
- that
- the
- theory
- there
- thoughtful
- tigra
- time
- timing
- to
- today
- toddler
- together
- tomorrow
- toms
- Torrente
- Total
- Tottenham
- tourer
- towers
- toyota
- transmission
- transporter
- trikot
- trolls
- true
- tumblr
- turks
- turning
- Tviberg
- twain
- twelfth
- twins
- tyga
- Tyler
- ucrânia
- UEFA
- ulang
- ultra
- unique
- united
- urdu
- us
- used
- using
- usyk
- va
- vaccine
- Valencia
- Vallé
- value
- van
- vbscript
- Vendedor
- verse
- verses
- Verstappen
- veterans
- VfB
- video
- villa
- vince
- volkswagen
- waiting
- wallpaper
- wallpapers
- Wanderers
- watt
- wayne
- web
- weber
- wedding
- what
- whats
- when
- where
- wife
- wiki
- wikipedia
- wild
- William
- willie
- willowgrove
- wimbledon
- win
- wise
- wishes
- with
- witty
- Woodley
- word
- worth
- XY
- yamamoto
- year
- yes
- yogyakarta
- your
- youre
- yourself
- youth
- youtube
- yuval
- zandvoort
- zappa
- zemmour
- ziglar
- zodiac
- zombieland
- zverev
Featured Post
Falling In Love With Your Cousin Quotes
Falling In Love With Your Cousin Quotes . Keep in mind that all of the great memories that we shared are among the best moments. “cousins ar...
